Minaris Regenerative Medicine has over 20 years of experience as a specialized cell and gene Contract Development and Manufacturing Organization (CDMO). We perform GMP manufacturing of all types of cell products and have a clear understanding that each project has unique characteristics and needs.
We offer you comprehensive manufacturing experience fully compliant with relevant regional regulatory requirements of our global facilities in the USA (FDA), Germany (EU-wide, EMA), and Japan (PMDA).
Learn more about how our experts can support your GMP manufacturing project starting from technology transfer to clinical manufacturing, and ultimately to commercial production of your ATMPs.

Minaris Regenerative Medicine has completed more than 100 successful technology transfers for cell therapy products. We take a systematic and strategic approach to transfer your technical knowledge/experience, specifications, procedures, and other critical data to our organization. If required, we offer in-house process development to adapt your process to GMP conditions or to further optimize the process.
Our goal is to establish a GMP-compliant manufacturing process based on state-of-the-art technologies that is required for your respective clinical stage and that meets all regulatory requirements (FDA, EMA, or PMDA).
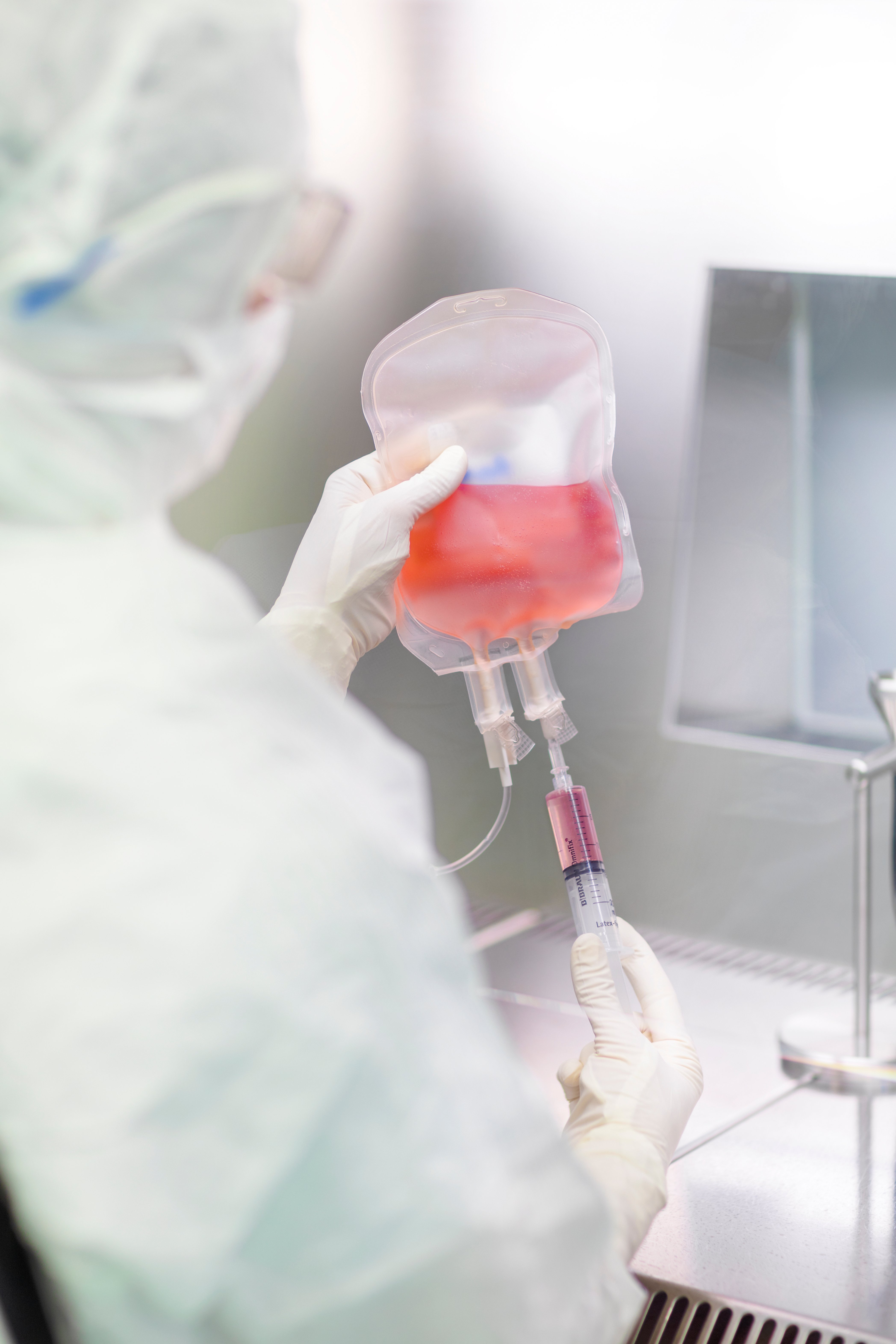
Minaris Regenerative Medicine listens to your specific needs, challenges, and goals, and creates a customized cell-based therapy clinical contract manufacturing program designed for a fruitful and valuable partnership.
We help you progress through the various stages of clinical development and optimize your manufacturing process towards commercial viability by providing an ideal environment.
Our GMP manufacturing capabilities include:

Minaris Regenerative Medicine has valuable experience in contract manufacturing of commercially available cell therapy products. Whether you are anticipating regulatory approval, or already have a marketed product, we have the commercial manufacturing capabilities to support your needs.
All our facilities are situated close to international airports, so as a known consignor for aviation security, we can offer fast, safe, and reliable product supply.